HyBryte™ Cutaneous T-Cell Lymphoma (CTCL) Treatment
| Preclinical | Phase 1 | Phase 2 | Phase 3 | NDA Review | Market |
|---|---|---|---|---|---|
|
Preclinical
|
Phase 1
|
Phase 2
|
Phase 3
|
NDA Review
|
Market
|
Orphan and/or Fast Track Designation
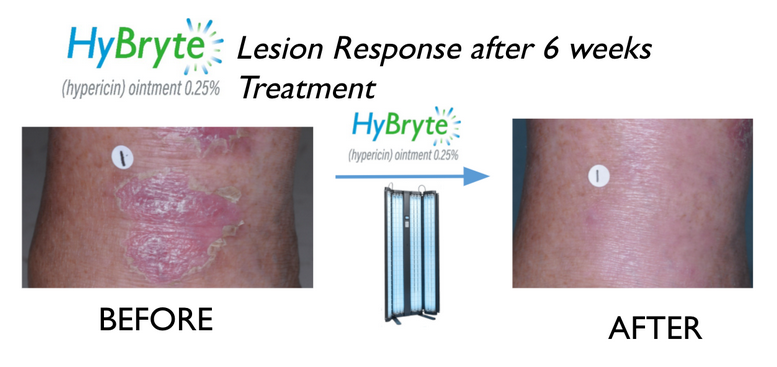
Cutaneous T-Cell Lymphoma treatment for early stage CTCL lesions is complicated given the side effects of many commonly used approaches. HyBryte™ (synthetic hypericin or SGX301) may provide a safer, more convenient alternative. It is a topical ointment which is applied to the lesions and then activated by safe visible fluorescent light. HyBryte™ is being tested as a photodynamic light therapy with an enhanced safety profile and a treatment regimen that may be completed in as little as 6 weeks. Our pivotal Phase 3 clinical study (the “FLASH” study) for CTCL has completed enrollment showing a statistically significant outcome after 6 weeks treatment (p=0.04) with continued improvement to 40% response rate after 12 weeks treatment (p<0.0001).
Background on Cutaneous T-Cell Lymphoma
The majority of patients with CTCL have early stage disease (called Mycosis Fungoides) which, with careful disease management, may never progress to later stages of disease. CTCL affects about 6% of non-Hodgkin’s lymphoma patients.
20,000
Approximately 20,000 patients in the US are believed to have this chronic disease with similar prevalence in Europe and significant incidence in Asia as well. CTCL is caused by the migration of malignant T-cells to the surface of the skin.
The characteristic lesions, tumors and plaques associated with CTCL can be extremely uncomfortable and treatment for cutaneous t-cell lymphoma is believed to slow disease progression.
Current CTCL Treatments
There is NO FDA-approved first-line therapy for CTCL. Every potential treatment is either used off-label (i.e., it wasn’t specifically approved for use in CTCL) or is supposed to be used when other cutaneous t-cell lymphoma treatments fail. There is a high risk of serious side effects with most current therapies. Because early-stage CTCL is a disease which patients can live with for a long time, it is very important to prevent the accumulation of toxic side effects (such as the risk for melanoma) while maintaining effective treatment of the lesions. Living with CTCL means using a number of different therapies over a lifetime, while managing the accumulating toxic side effects.
The FLASH study has been completed. Stay tuned for more information!
Our Approach: HyBryte™
HyBryte™ appears to be a SAFE and effective photodynamic therapy using visible fluorescent light. It is designed to be a front line treatment for CTCL. HyBryte™ may enable patients to undergo more treatments to manage their disease while accumulating significantly fewer risks/toxicities.
What is Photodynamic Light Therapy?
Photodynamic light therapy is a two part treatment. First a drug is given and can be an ointment or a pill, for example. Then the drug is activated by light.
Photodynamic therapy in general can use many different kinds of light – UV A, UV B, fluorescent light, lasers, etc. The specific light used depends on the drug molecule and how much energy is needed to activate it.
What is HyBryte™ and how does it work in CTCL?
HyBryte™ is a photodynamic therapy using synthetically manufactured hypericin in an ointment combined with visible fluorescent light.
Hypericin is one of the most photoactive compounds known – it is easily activated with relatively low energy light. This makes it ideal for photodynamic therapy because it can be activated with fluorescent light, instead of UV A or UV B light, which are associated with increased cancer risks.
Mechanism of Action
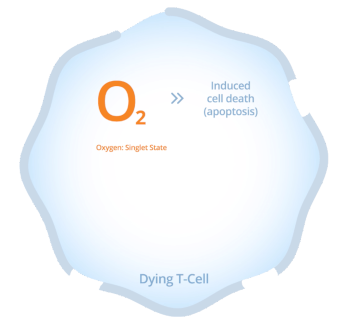
Synthetic hypericin, the active ingredient in HyBryte™, tends to accumulate in T-cells. Once the hypericin is in the T-cells, it can be activated by safe, visible fluorescent light.
When synthetic hypericin is activated it creates oxygen radicals which subsequently cause cellular toxicity, killing the targeted T-cells.
As the concentration of both the drug inside the cell as well as the light intensity increases, cell death in the targeted cells increases.
This results in clearing of the CTCL lesion which is thought to decrease the risk of disease progression. Because the synthetic hypericin is applied in an ointment, it only targets the T-cells in the skin layer, and does not circulate within the body, further reducing toxicity.
Importantly, the mechanism by which the activated hypericin kills the T-cells does NOT involve mutation of the DNA of the cells. Therefore, mutagenic risk is minimized. Similarly, use of fluorescent light reduces the carcinogenic risk, relative to other potential light sources used.
More details on the mechanism of action of HyBryte™ can be found in our Reference Literature.
Clinical Studies & Commercialization
Following up on the completed Phase 1, 2 and 3 studies evaluating HyBryte as a treatment for CTCL, Soligenix is collaborating with Dr. Ellen Kim from the University of Pennsylvania to conduct an open-label clinical study evaluating HyBryte therapy as a treatment for CTCL where patients may be treated for up to 1 year. The study is partially funded by the US Food & Drug Administration via an Orphan Products Development grant (1R01FD007262-01A1).
Further information on the HyBryte™ program can be found in this interview with our Chief Medical Officer, Richard Straube, MD.
HyBryte™ has demonstrated positive and statistical significant results in Phase 1, 2 and 3 clinical studies. Soligenix is currently working with the FDA and EMA to design a second, confirmatory Phase 3 study to support potential marketing approval.
Interested in partnering with us for commercializing in specific geographical regions?
Previous Clinical Studies with HyBryte™
HyBryte™ has demonstrated safety in a Phase 1 clinical study in healthy volunteers. In two Phase 2, clinical studies in patients with CTCL, the drug was safe, well tolerated, and effective in ameliorating the skin lesions.
Other Potential Uses for Synthetic Hypericin
Synthetic hypericin may also be useful in psoriasis. Psoriasis is characterized by the migration of T-cells to the skin surface. However, unlike in CTCL, the T-cells in psoriasis are not malignant. Psoriasis affects over 7 million adults in the US. Photodynamic therapy is a frequently employed initial therapy for psoriasis, despite the need for ultraviolet light exposure and its attendant risk of melanoma and non-melanoma skin cancer. The Phase 1/2 and Phase 2a clinical studies showed that hypericin and visible light phototherapy is also potentially effective in treating these lesions.
Regulatory Status
Synthetic hypericin sodium, the active ingredient in HyBryte™, has Orphan Drug designation in the United States for the treatment of T-cell lymphoma and CTCL and in Europe for CTCL.
HyBryte™ has Fast Track designation for the treatment of cutaneous t-cell lymphoma (CTCL) in the United States.
HyBryte™ has Promising Innovative Medicine designation and has been awarded an “Innovation Passport” from the United Kingdom Medicines and Healthcare products Regulatory Agency.
Intellectual Property
Soligenix has a worldwide intellectual property position on the use of photoactivated hypericin.
Orphan Drug designation also confers 7 years market exclusivity in the US and 10 years in Europe, if approved.
Our pipeline focuses on orphan and unmet medical need across a range of indications
CTCL Scientific Advisory Board
Our scientific advisory board for CTCL has decades of experience in the biopharmaceutical industry, which includes unique expertise in developing orphan/rare disease therapies. All content on this page has been reviewed by our team of experts.