SGX942 for Oral Mucositis Treatment
| Preclinical | Phase 1 | Phase 2 | Phase 3 | NDA Review | Market |
|---|---|---|---|---|---|
|
Preclinical
|
Phase 1
|
Phase 2
|
Phase 3
|
NDA Review
|
Market
|

Oral mucositis is a debilitating side effect of cancer treatment. There is no FDA-approved treatment for oral mucositis in patients undergoing treatment for solid tumors. Dusquetide (an Innate Defense Regulator and the active ingredient in SGX942) is unique in its mechanism addressing the underlying innate immune dysfunction that contributes to the severity and duration of mucositis. Administered twice weekly by a brief 4-minute IV infusion during chemoradiation treatment while patients are otherwise present at their clinician’s office or hospital, SGX942 may be a clinically convenient and safe approach to mitigating oral mucositis. Our pivotal Phase 3 clinical study (“DOM-INNATE”) for oral mucositis in head and neck cancer patients has completed enrollment.
The primary endpoint of median duration of SOM did not achieve the pre-specified criterion for statistical significance (p≤0.05); although biological activity was observed with a 56% reduction in the median duration of SOM from 18 days in the placebo group to 8 days in the SGX942 treatment group. Despite this clinically meaningful improvement, the variability in the distribution of the data yielded a p-value that was not statistically significant.
Background on Mucositis
Mucositis is the damage done to the lining of the mouth and gut by cancer treatment regimens. Severe Oral Mucositis (SOM) occurs when the damage to the mouth is so severe a patient’s ability to eat and/or drink is compromised. For patients already undergoing cancer treatment, an added inability to eat and/or drink means they are more likely to be dehydrated and malnourished, and are more susceptible to life-threatening infections. On average, patients with mucositis have a reduced quality of life, take more pain medications such as opioids, and spend more time in hospital. For some patients, SOM causes them to stop their cancer treatment, potentially impacting long term survival.
Mucositis of either the mouth or gut occurs in most different cancer treatment regimens. In head and neck cancer, 95% of patients have some form of oral mucositis and over 70% have SOM.
Current Treatments for Oral Mucositis
There is NO FDA-approved drug for oral mucositis treatment for patients with solid tumors.
Some gels and rinses are marketed for OM as “devices” meaning they did not have to prove efficacy to the FDA or other regulatory agency.
Recommended oral mucositis treatments include:
- ice chips (cryotherapy),
- preventive and supportive dental care,
- strong oral cleaning procedures and
- gels/rinses which coat the surface of the mouth and contain pain medication (for example, MuGard, caphosol, etc.)
Despite these procedures, SOM still occurs in large numbers of patients, impacting quality of life, increasing the likelihood of hospital admissions and an increasing likelihood of stopping all anti-cancer treatment, potentially impacting long term survival.

The DOM-INNATE study evaluating SGX942 treatment of oral mucositis in head and neck cancer has completed throughout the US and Europe.
Our Approach to Oral Mucositis Treatment: SGX942
SGX942 is a rapid 4-minute infusion administered twice per week during chemotherapy and/or radiation treatment and is under investigation to reduce the duration and severity of severe oral mucositis.
What is SGX942?
SGX942 is an intravenous formulation of the Innate Defense Regulator, dusquetide, for the treatment of SOM.
What are Innate Defense Regulators?
Innate Defense Regulators (IDRs) are a new class of compound that change the response of the innate immune system by decreasing inflammation and increasing anti-infective and tissue healing activities.
IDRs modulate the response of the innate immune system to any insult – including infection and tissue damage. Their main modes of action are to control inflammation while enhancing tissue healing and anti-infective action.
Oral mucositis is driven by dysfunction of the innate immune system, and IDRs reset the innate immune response – mitigating the damaging inflammatory response caused by the chemotherapy and radiation.
Dusquetide is the first IDR to be tested in the clinic.
What is dusquetide?
Dusquetide is a drug composed of 5 amino acids, naturally occurring molecules which make up the proteins in your body. Therefore, metabolism of dusquetide results in additional building blocks for your body and is not known to interfere with any other drugs you may be taking.
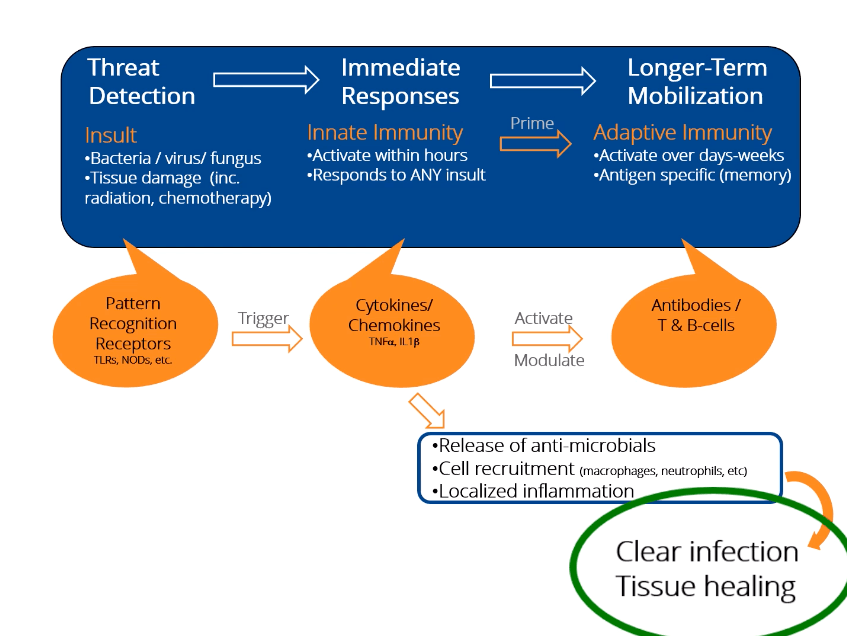
What is Innate Immunity?
The innate immune system is the pre-programmed response arm of the immune system. By two years of age, most people’s innate immune system is fully functional. In order to identify threats, the innate immune looks for common “response patterns” or signals in different types of tissue damage and infections.
The innate immune system is your body’s rapid response team to deal with immediate threats (infection, wounds, radiation damage, etc.).
How Innate Immunity Works
- The innate immune system is characterized by many different “damage detectors” (receptors) that people are born with.
- The damage detectors respond immediately (there is no need to wait for the immune system to ‘learn’ anything). This is the primary difference between innate immunity (immediate response) and adaptive immunity (learned response of T-cells and antibodies where the learning process can take days or weeks).
- Triggering the damage detectors launches both inflammatory responses as well as tissue healing and anti-infective responses.
- Regardless of the damage detectors activated, only a few proteins inside the cell are responsible for dictating the immediate response of the innate immune system.
Mechanism of Action
Unlike other attempts to modulate the innate immune response, IDRs, like dusquetide, are unique because they target the intracellular control pathways including the protein p62 of the innate immune system – changing the character of the innate immune response. They decrease or control the inflammatory component and increase the tissue healing or anti-infective component. The effect on the innate immune response is immediate (within 30 minutes) and long-lasting (up to 5 days).
Clinical Studies & Commercialization

We are currently conducting clinical research with SGX942 in oral mucositis in head and neck cancer patients.
DOM-INNATE Results
The DOM-INNATE study enrolled 268 patients, 266 of which were included in the intent-to-treat (ITT) population. The ITT population includes all patients that received at least one dose of study drug.
The primary endpoint of median duration of SOM did not achieve statistical significance (p≤0.05); although biologic activity was clearly observed with a 56% reduction in the median duration of SOM from 18 days in the placebo group to 8 days in the SGX942 treatment group. Other secondary endpoints supported the biologic activity of dusquetide as well, including a statistically significant 50% reduction in the duration of SOM in the per-protocol (PP) population, which decreased from 18 days in the placebo group to 9 days in the SGX942 treatment group (p=0.049), consistent with the findings in the Phase 2 trial.
210 patients were in the PP population. The PP population includes all patients that followed the important protocol procedures with respect to drug administration.
Both ITT and PP populations saw clinically meaningful changes. The time for which the patients experienced severe oral mucositis was reduced by approximately 50%. The changes in the PP population were statistically significant.
Incidence of severe oral mucositis also improved in both populations, as well as other secondary endpoints.
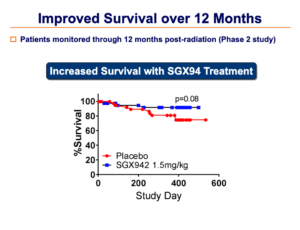
These Phase 3 results were very consistent with the Phase 2 results, particularly in terms of the decrease in duration of oral mucositis.
With the benefit of a robust preclinical and clinical data package for SGX942, we now will be analyzing the combined Phase 2 and 3 datasets to design a second Phase 3 study and will look to identify a potential partner(s) to continue this development program.
Interested in partnering with us?
Previous Clinical Studies with SGX942
SGX942 has demonstrated safety in a Phase 1 clinical study in 84 healthy volunteers.
In a Phase 2, placebo-controlled, clinical study in 111 head and neck cancer patients, SGX942 was safe, well tolerated, and effective in reducing the duration of severe oral mucositis.
Further information on the SGX942 program can be found in this webcast discussing our Phase 2 results.

Additional benefits in terms of increased tumor resolution and increased survival were also observed.


Other Potential Uses for Dusquetide
SGX942 is expected to be equally effective across all mucositis – whether in the mouth or gut and whether caused by either chemotherapy or radiation.
Dusquetide has also shown preclinical efficacy in controlling and suppressing tumor growth, including with breast cancer cell lines.
As an Innate Defense Regulator, dusquetide (the active ingredient in SGX942) has a number of potential applications.
See our SGX943 page for more details about infectious disease applications.
Regulatory Status
SGX942 has Fast Track designation for the treatment of oral mucositis in the United States.
SGX942 for the treatment of oral mucositis has Promising Innovative Medicine designation from the United Kingdom Medicines and Healthcare products Regulatory Agency.
MHRA has indicated that the DOM-INNATE study could serve as the first of two Phase 3 studies required to support potential marketing authorization, assuming the second Phase 3 clinical trial achieves the required level of statistical significance in its primary endpoint.
Intellectual Property
Soligenix has a strong worldwide intellectual property position on the composition of matter of dusquetide and related analogs and on therapeutic use in mucositis, as well as other indications.
Our pipeline focuses on orphan and unmet medical need across a range of indications
Oral Mucositis Scientific Advisory Board
Our scientific advisory board for Oral Mucositis has decades of experience in the biopharmaceutical industry, which includes unique expertise in developing orphan/rare disease therapies. All content on this page has been reviewed by our team of experts.