
SGX203 for Pediatric Crohn’s Disease
| Preclinical | Phase 1 | Phase 2 | Phase 3 | NDA Review | Market |
|---|---|---|---|---|---|
|
Preclinical
|
Phase 1
|
Phase 2
|
Phase 3
|
NDA Review
|
Market
|
Orphan and/or Fast Track Designation
A New Potential Treatment for Mild to Moderate Pediatric Crohn’s Disease
The symptoms of Pediatric Crohn’s disease can come and go, with disease “flares” resulting in severe symptoms and the need for symptom-mitigating treatment. These disease flares are usually treated with systemic steroids, but treatment timelines are as limited as possible given the significant toxicity of steroids, especially in children. There are currently no FDA-approved treatments for mild to moderate Crohn’s disease, with systemic steroid treatment used on an “off-label” basis under physician orders. SGX203 is an oral steroid formulation which will coat the gastrointestinal tract to reduce inflammation but which is not as likely to circulate in the blood, reducing the systemic side effects of steroid treatment. SGX203 has the potential to be the first approved therapy for mild to moderate Crohn’s disease in children.

Background on Pediatric Crohn’s Disease
Pediatric Crohn’s Disease is a subset of disease with primary onset in patients less than 20 years of age. Approximately 20% of Crohn’s disease patients have pediatric onset.
Pediatric Crohn’s disease is distinguished from the adult onset version of the disease by the involvement of the entire gastrointestinal tract, whereas adult patients generally have disease limited to the distal or lower GI tract.
Crohn’s disease is an inflammatory condition of the gut and it can wax and wane over time, with increases in disease severity labeled as “flares”.
Crohn’s Disease is associated with abdominal pain, diarrhea, rectal bleeding, poor ability to absorb nutrients from food and weight loss. In children, this often translates into a lack of growth. Moreover, the inflammation can cause changes in the structure of the intestine, requiring corrective surgery.
There are over 160,000 children and adolescents with Crohn’s disease worldwide.
Current Pediatric Crohn’s Disease Treatments
There is NO approved therapy for mild to moderate Crohn’s disease. Treatment is limited to off-label use of steroids and other anti-inflammatory treatments and second-line use of biologics.
Prednisone (steroid) Treatment
When changes in nutrition and lifestyle are inadequate to control disease flares, immediate treatment is often initiated with high doses of prednisone.
Prednisone is a steroid that helps to control the inflammation. However, steroids have a number of associated side effects, including causing high blood pressure, glucose intolerance, reduced bone mass, cataracts, decreased growth and increased risk of infections. Some of these side effects further exacerbate complications from the disease itself such as lack of growth. Because prednisone is available throughout the body (systemically), all these side effects occur. Due to the severity of side effects, treatment is kept as minimal as possible.
Unfortunately, steroid use can also lead to steroid dependence, such that stopping treatment with the steroids results in immediate disease flare ups.
Biological Treatment
There are two FDA approved treatments for severe Crohn’s disease that have failed conventional (e.g., steroid) therapy. These treatments suppress specific inflammatory signaling in the body.
Both products are associated with BLACK BOX warnings for causing cancer (e.g., lymphoma) and increasing risk of life-threatening infection.
- Humira® (Adalimumab) is given as an injection once every two weeks.
- Remicade®(Adalimumab) is given as an injection once every two weeks.
Our Approach to Pediatric Crohn’s Disease Treatment: SGX203
SGX203 is designed as a topical treatment for the gut. The active ingredient is beclomethasone 17,21-dipropionate (BDP).
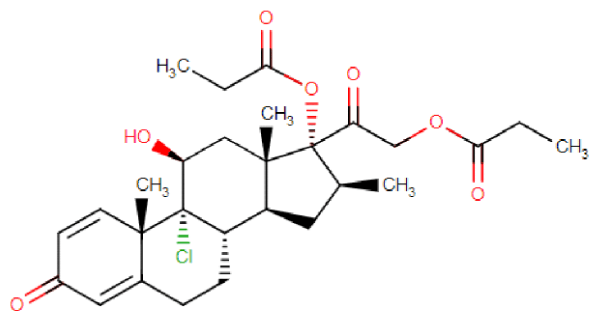
BDP is a steroid which can reduce local inflammation but has limited ability to penetrate into the bloodstream.
By providing a formulation of BDP which coats the inside of the gut with steroid, but isn’t absorbed into the bloodstream, the systemic side effects of steroid treatment may be reduced.
SGX203 may be a safer alternative for first-line treatment of mild to moderate pediatric Crohn’s Disease.
How is SGX203 Delivered?
SGX203 is a combination of two pills, both containing the active ingredient BDP. One of the pills is designed to dissolve in the stomach, releasing BDP to coat the upper gastrointestinal tract. The other pill is designed to dissolve in the intestine, coating the bottom half of the gastrointestinal tract.
Once a disease flare occurs, SGX203 can be taken twice per day, starting at a higher dose and then reducing once the symptoms begin to fade.
What is Known About BDP?
Beclomethasone dipropionate (BDP) is a very well-known steroid that has been used in inhaled products for asthma, in nasal sprays for rhinitis and in skin creams for rashes. It has been used in children and adults for over 40 years. There is a lot of information known about the safety and efficacy of BDP.
Mechanism of Action
As with all corticosteroids, BDP binds to the corticosteroid receptor and has anti-inflammatory action, suppressing the infiltration of inflammatory cells to the local site of action.
The distinguishing feature of BDP is that it does not travel throughout the body easily, meaning that it has a local action without inducing as many side effects throughout the body. This also means that the BDP must be delivered directly to where it is needed – which is reflected in the unique oral formulation that is used in SGX203. More details on the mechanism of action of SGX203 can be found in our Reference Literature.
Clinical Studies & Commercialization
Because there is no approved front-line therapy for pediatric Crohn’s disease, significant discussion was undertaken with FDA to identify an acceptable and feasible adaptive Phase 3 clinical trial design. It is anticipated that only one trial of this design can be run in children, since once efficacy is demonstrated it would be unethical to run another controlled trial.
The SGX203 Phase 3 trial is ready to proceed pending partnership or funding.
Interested in partnering with us for this program?
Phase 3 Trial Design
The Phase 3 trial is a double-blind, randomized, controlled, multi-national trial that will enroll approximately 150 subjects, 6-17 years of age, with endoscopically proven mild to moderate Crohn’s disease. The trial will compare the rates of improvement of the signs and symptoms of Crohn’s disease after 8 weeks of treatment among subjects randomized to one of three SGX203 dose groups (split 60:30:60 among the lowest, middle and highest dose of SGX203). Subjects will be followed for an additional 6 months after the completion of treatment.
Entry criteria for the trial are the presence of Crohn’s disease symptoms (abdominal pain and/or diarrhea) associated with laboratory blood tests showing evidence of a disease flare.
The primary clinical efficacy endpoint of the trial will compare the percentage of subjects in each of the three dose groups having resolved their signs and symptoms after an 8-week course of treatment.
An adaptive design will be employed in which an independent Data Monitoring Committee will review the efficacy data after approximately 90 subjects have completed treatment and determine if the trial size requires adjustment based on the actual event rate, halted for futility, or overwhelming efficacy.
Previous Clinical Studies with SGX203
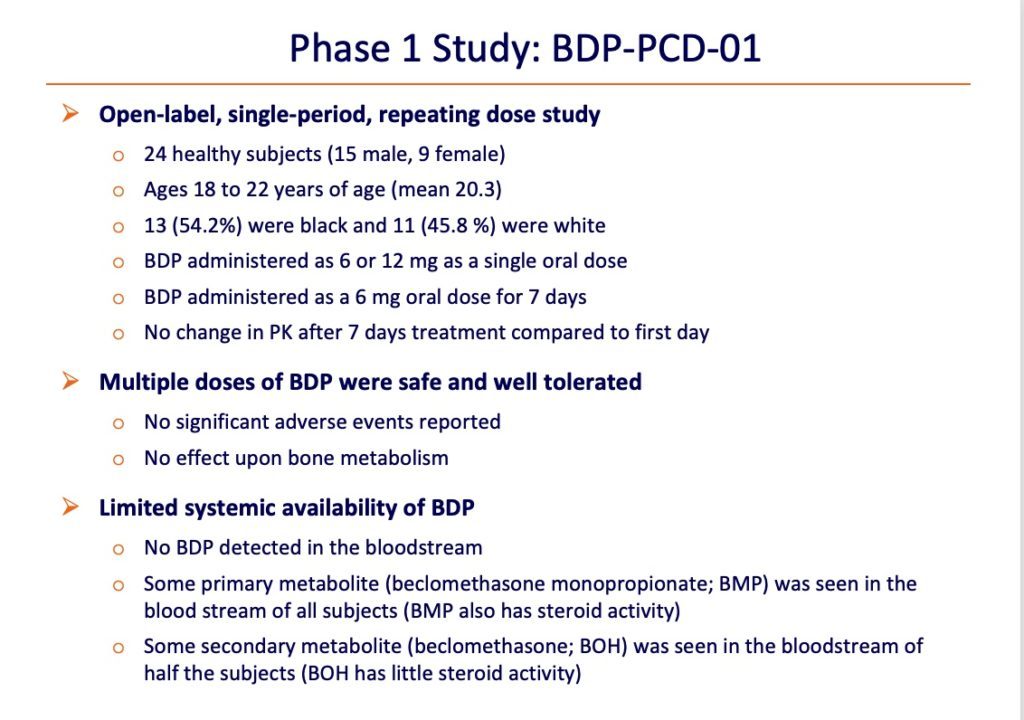
The safety of SGX203 was assessed in a Phase 1 study in 24 male and female healthy adolescents and young adults. SGX203 was found to be safe, to have no effect on bone metabolism (a common problem with systemic steroids) and to have limited systemic availability.
Other Potential Uses for BDP
A topical oral anti-inflammatory with action limited to the gastrointestinal tract may be useful in a number of other inflammatory conditions, including inflammation occurring as the result of radiation exposure.
Acute Radiation Syndrome (OrbeShield)
The SGX203 formulation is also being explored in the context of Acute Radiation Syndrome, as a potential therapy in the case of a mass-casualty nuclear accident or attack.
Radiation Enteritis
Inflammation of the gut after radiation therapy is also a common occurrence in the context of cancer treatment. In particular, radiation enteritis is a known side-effect of cancer treatment of bladder, uterus, cervix, rectum, prostate and vagina and in all cases, anti-inflammatory treatment of the gut lining may reduce the damage to the gut.
Symptoms of acute radiation enteritis include nausea, vomiting, abdominal pain and rectal bleeding. While the symptoms of most patients may fade a few weeks or months after radiation treatment, 20% of patients may go on to develop chronic radiation enteritis. There are over 100,000 patients annually in the US receiving abdominal or pelvic radiation treatment for cancer who are at risk of developing acute and chronic radiation enteritis. Oral BDP consists of the delayed release tablet containing BDP, specifically targeting the lower gastrointestinal tract.
Regulatory Status
SGX203 has Orphan Drug designation for the treatment of Pediatric Crohn’s disease in the United States.
SGX203 has Fast Track designation for the treatment of Pediatric Crohn’s disease in the United States.
Intellectual Property
Soligenix has a strong worldwide intellectual property position on the proprietary formulation of BDP as an oral topical treatment for the gastrointestinal tract in pediatric Crohn’s disease and related inflammatory conditions of the gut.
In addition, Orphan Drug designation gives 7 years of marketing exclusivity for SGX203 in the US, once approved.
Our pipeline focuses on orphan and unmet medical need across a range of indications
Pediatric Crohn’s Disease Scientific Advisory Board
Our scientific advisory board for Pediatric Crohn’s disease has decades of experience in the biopharmaceutical industry, which includes unique expertise in developing orphan/rare disease therapies. All content on this page has been reviewed by our team of experts.
Meet the Pediatric Crohn’s disease scientific advisory board
Interested in helping us conduct the Phase 3 trial?
Come visit our partnering page