SGX945 for Aphthous Ulcers of Behçet’s Disease
| Discovery | Preclinical | Phase 1 | Phase 2 | Phase III |
|---|---|---|---|---|
|
Discovery
|
Preclinical
|
Phase 1
|
Phase 2
|
Phase III
|
Oral and Genital Ulcers are the Hallmarks of Behçet’s Disease
Behçet’s Disease is characterized by oral and genital lesions which are recurrent, extremely painful and prone to scarring. These lesions significantly impact Quality of Life and productivity. These oral and genital (aphthous) ulcer flares can be triggered by a variety of environmental and other factors, and management of the ulcer flares is an important component of overall lifestyle management. There is only one approved treatment for oral ulcers, which both must be taken continuously and is logistically challenging (due to cost). While other treatments are commonly used, none are foolproof and breakthrough ulceration is common. Dusquetide (an Innate Defense Regulator and the active ingredient in SGX945) is unique in its mechanism addressing the underlying innate immune dysfunction that contributes to the severity and duration of ulceration, as well as providing anti-infective and wound-healing properties. Administered twice weekly by a rapid 4-minute IV infusion during ulcerative flares, SGX945 is a clinically unique and safe approach to mitigating aphthous ulcers. Our phase 2 clinical study evaluating dusquetide in Behçet’s Disease is planned to initiate in 2024.
Background on Behçet’s Disease
Behçet’s Disease is a rarely occurring disease, more common along the “Silk Road” region of the world, including Turkey, Iran, Japan and China. It is characterized by a vasculitis which can manifest in multiple organs in the body. Most patients are diagnosed with Behçet’s Disease on the basis of recurrent oral and genital ulcers, and mucocutaneous ulcers are the more common manifestation of the disease. Behçet’s Disease is believed to be an auto-immune disease with both genetic and environmental components. The resulting inflammatory cascade due to the vasculitis involves both the innate and adaptive immune system. Behçet’s Disease symptoms are most severe in young adulthood and generally ameliorate with age. While the aphthous ulcers of Behçet’s Disease are extremely painful they are not usually associated with long term damage; although scarring is a prevalent concern. More rarely, Behçet’s Disease can affect other organ systems (e.g., eyes, lungs, brain) and may become life-threatening. Although treatments exist, oral and genital flares continue to occur for many patients and have a significant effect on Quality of Life and productivity.
Current Treatments
Other than steroid use, there is only one specifically approved drug for treatment of Behçet’s Disease. Apremilast is specifically directed to the treatment of oral ulcers of Behçet’s disease and is meant to be used continuously to prevent oral ulcers.
There are other treatments which have been studied in randomized controlled trials for Behçet’s Disease. Despite available treatments, patients with Behçet’s Disease can continue to experience breakthrough oral and genital ulcers which are extremely painful and prone to scarring. These ulcers affect the Quality of Life and productivity of patients suffering from Behçet’s Disease.
Our Approach: SGX945
SGX945 is a rapid 4-minute injection administered twice per week to enhance the rate of resolution of aphthous ulcers, including oral and genital ulcers.
What is SGX945?
SGX945 is an intravenous formulation of the Innate Defense Regulator, dusquetide, for the treatment of aphthous ulcers of Behçet’s Disease.
What is dusquetide?
Dusquetide is a drug composed of 5 amino acids, naturally occurring molecules which make up the proteins in your body. Therefore, metabolism of dusquetide results in additional building blocks for your body and is not known to interfere with any other drugs you may be taking.
What is Innate Immunity?
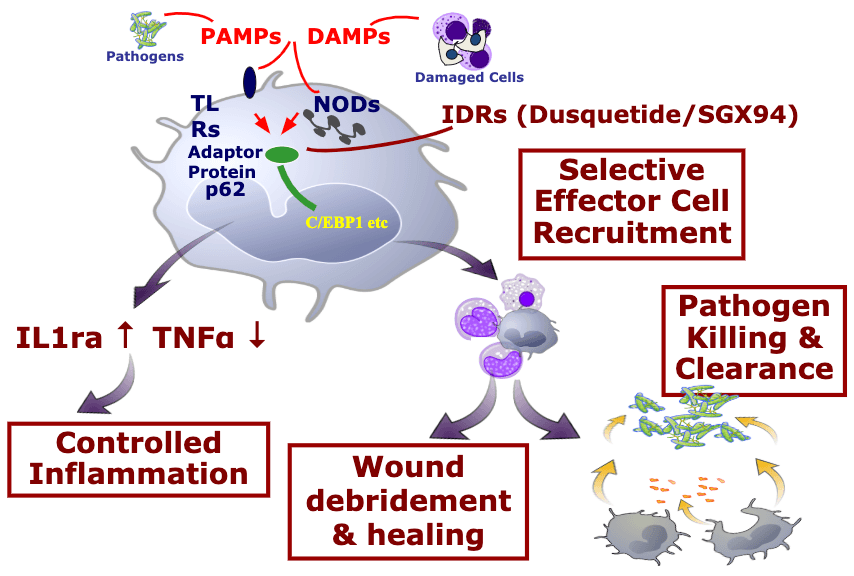
The innate immune system is the pre-programmed response arm of the immune system. By two years of age, most people’s innate immune system is fully functional. In order to identify threats, the innate immune system looks for common “response patterns” or signals in different types of tissue damage and infections.
The innate immune system is your body’s rapid response team to deal with immediate threats (infection, wounds, radiation damage, etc.).
How Innate Immunity Works
- The innate immune system is characterized by many different “damage detectors” (receptors) that people are born with
- The damage detectors respond immediately (there is no need to wait for the immune system to ‘learn’ anything). This is a primary difference between innate immunity (immediate response) and adaptive immunity (learned response of T-cells and antibodies where the learning process can take days or weeks).
- Triggering the damage detectors launches both inflammatory responses as well as tissue healing and anti-infective responses.
- Regardless of the damage detectors activated, only a few proteins inside the cell are responsible for dictating the immediate response of the innate immune system.
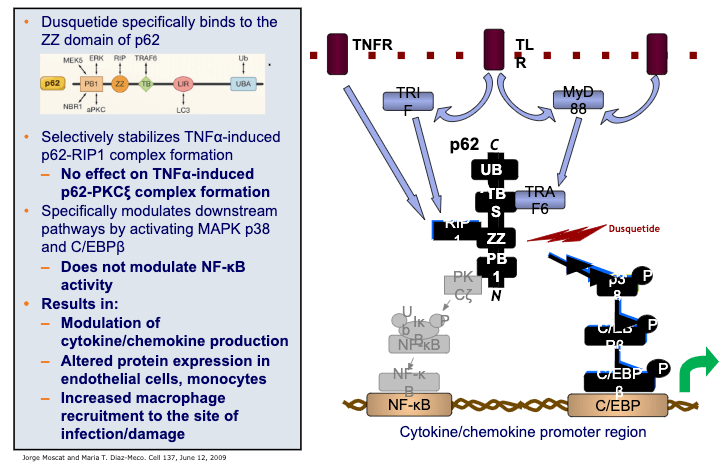
Mechanism of Action
Unlike other attempts to modulate the innate immune response, IDRs like dusquetide are unique because they target the intracellular control nodes including the protein p62 of the innate immune system – changing the character of the innate immune response. They decrease or control the inflammatory component and increase the tissue healing or anti-infective component. The effect on the innate immune response is immediate (within 30 minutes) and long-lasting (up to 5 days).
Clinical Studies & Commercialization
We are currently conducting clinical research with SGX945 in the treatment and resolution of aphthous ulcer flares in Behçet’s Disease. The Phase 2 trial is cleared to proceed by the US FDA and is intended to begin in 2024.
Interested in partnering with us for developing or commercializing SGX945 in specific geographical regions? Please see our Partnering Page.
Previous Clinical Studies with Dusquetide
Dusquetide has demonstrated safety in a Phase 1 clinical study in 84 healthy volunteers.
In a Phase 2, placebo-controlled, clinical study in 111 head and neck cancer patients, dusquetide was safe, well tolerated, and effective in reducing the duration of severe oral mucositis.
Additional benefits in terms of increased tumor resolution and increased survival were also observed.
In a Phase 3 placebo-controlled clinical study in 268 head and neck cancer patients, dusquetide was safe, well-tolerated and demonstrated biological response in reducing the severity of severe oral mucositis.
A Phase 2 study evaluating the potential utility of SGX945 (dusquetide) in the treatment of aphthous ulcer flares in Behçet’s Disease is planned to begin in 2024.
Other Potential Uses for Dusquetide
SGX942, which also contains dusquetide, is expected to be equally effective across all mucositis – whether in the mouth or gut and whether caused by either chemotherapy or radiation.
As an Innate Defense Regulator, dusquetide (the active ingredient in SGX942 and SGX945) has a number of potential applications.

Regulatory Status
SGX945 IND filed with the US FDA and the Phase 2 protocol has cleared.
SGX945 has FDA Fast Track designation for the treatment of Behçet’s Disease aphthous ulcer flares.
SGX942 has fast track designation for the treatment of oral mucositis in the United States.
SGX942 has Promising Innovative Medicine designation for the treatment of oral mucositis in the UK MHRA.
Intellectual Property
Soligenix has a strong worldwide intellectual property position on the composition of matter of dusquetide and related analogs and on therapeutic use in mucositis and Behçet’s Disease, as well as other indications.
Interested in participating in our Behçet’s Disease trial? Please contact us.